2-3 spatulas of calcium hydroxide in a 500ml stock bottle and make up with distilled water. Mix well, then allow the solids to settle. If desired solids can be filtered off, but this is not necessary.
Limewater is saturated calcium hydroxide Ca(OH)2.
Add (solid) calcium hydroxide to water until no more will dissolve. Let it settle, preferably overnight, so that the limewater solution is clear.
Store in dedicated 2.5L Winchesters and leave some excess solid at the base.
When you decant, the solid will start to mix with the clear liquid, so having two containers is handy.
25g of calcium hydroxide solid into 1000ml of distilled water, in a Winchester. Shake, allow solid to settle and draw off the clear liquid. Every now and again give it a shake and let settle again. You can keep topping it up with water.
Dissolve calcium hydroxide into a beaker containing 3/4 of the total final volume and agitate using a magnetic stirrer for about 24h. Cover the beaker with glad wrap or watch glass, to avoid oxidation.
Once fully dissolved, makeup to final volume and filter the solution to get rid of any undissolved calcium hydroxide. Transferred solution into a sealed container.
Lime water is approximately a 0.02M solution of Calcium Hydroxide.
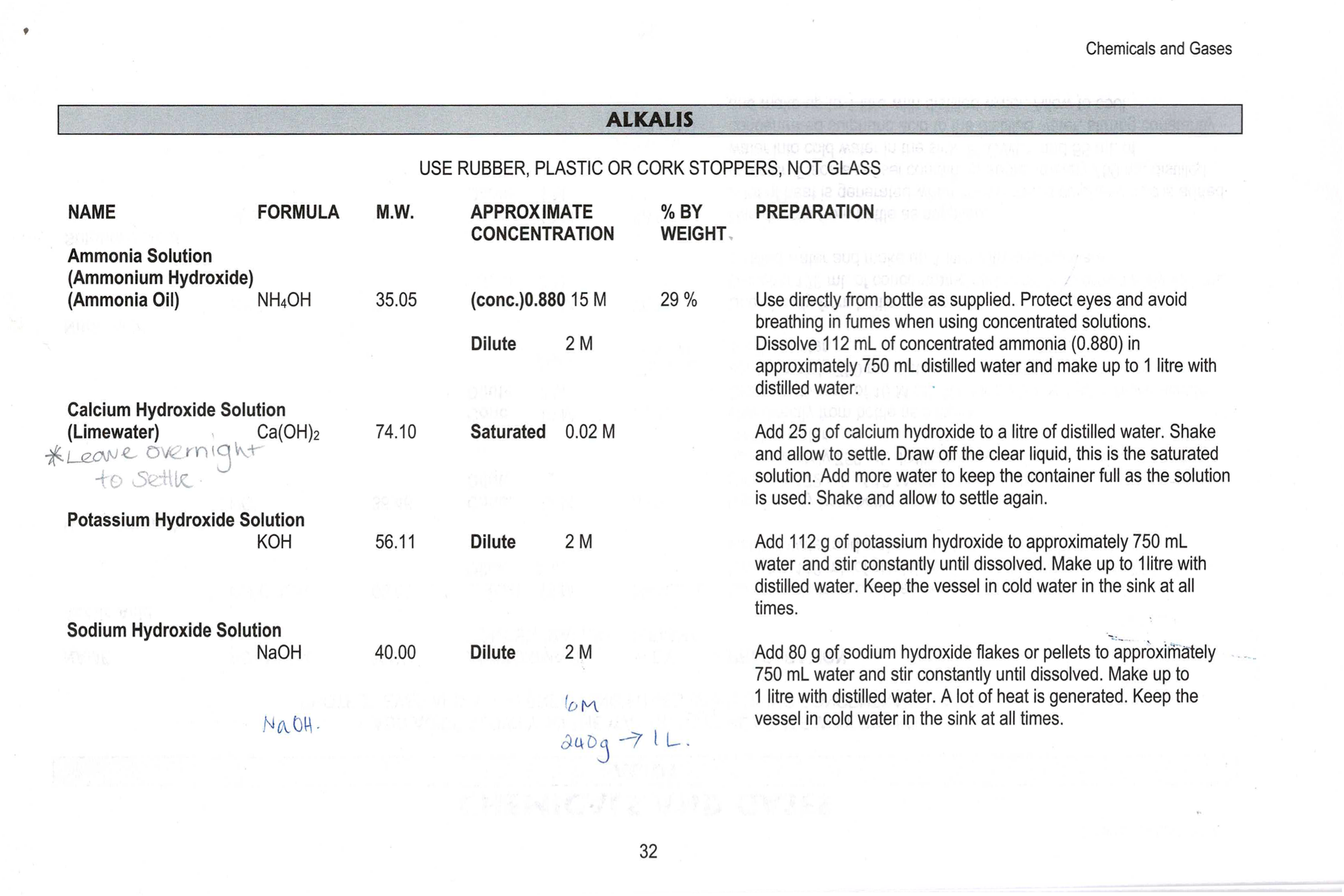
Add 25g of calcium hydroxide to a litre of water. Shake and allow to settle.
Draw off the clear liquid, this is the saturated solution.
Add more water as the solution is used, to the keep the container full. Shake and allow to settle again.
The allows the original solution to be used multiple times.
From the lab Bible
The Laboratory – A Science reference & preparation manual for schools.
B. Dungey